
APAC Intracranial Pressure Monitoring Devices Market Outlook to 2030
Region:Asia
Author(s):Meenakshi Bisht
Product Code:KROD8183
December 2024
94
About the Report
APAC Intracranial Pressure Monitoring Devices Market Overview
- The Asia Pacific Intracranial Pressure Monitoring Devices Market is valued at USD 480.5 million based on a five-year historical analysis. This market is driven by the increasing prevalence of neurological disorders such as traumatic brain injuries and strokes, along with rising demand for non-invasive monitoring techniques. Additionally, advancements in medical technologies, along with a surge in healthcare spending, have significantly contributed to the growing adoption of these devices across major healthcare institutions in the region.
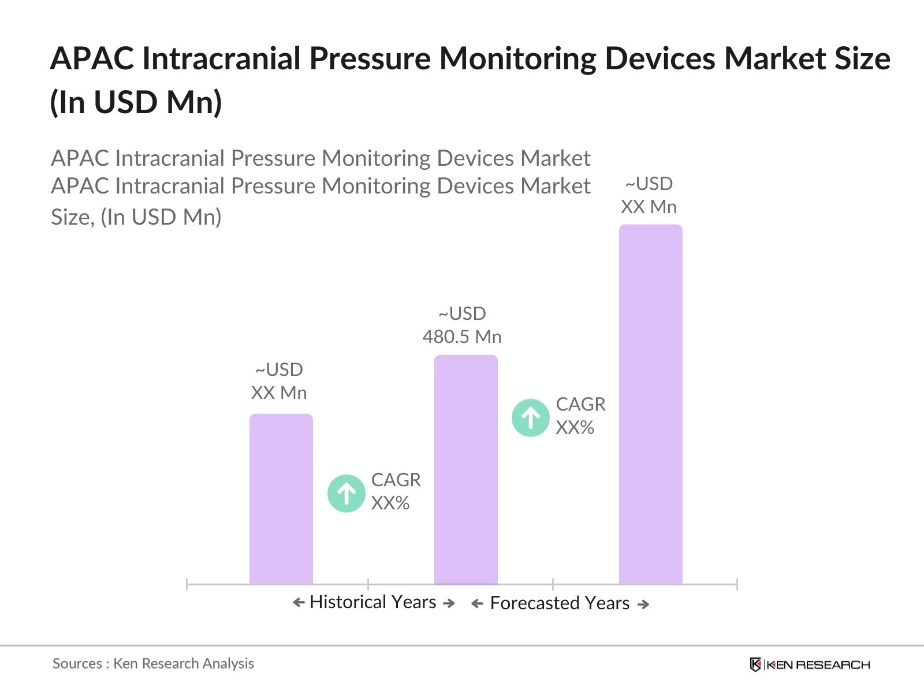
- Countries like China, Japan, and India dominate the Asia Pacific Intracranial Pressure Monitoring Devices market. This dominance is attributed to their large population base, increasing investments in healthcare infrastructure, and rising incidences of neurological disorders. China and Japan, in particular, have well-established healthcare systems and are leading in the adoption of advanced medical technologies, which has positioned them as market leaders. Moreover, government initiatives aimed at improving healthcare access in these countries have further bolstered their market positions.
- The regulatory pathways for medical devices, including ICP monitors, vary across APAC countries, making it essential for manufacturers to navigate different approval processes. In 2023, the Australian Therapeutic Goods Administration (TGA) medical device applications, with a focus on ensuring that devices meet stringent health and safety standards. These regulatory hurdles can delay market entry but also ensure that the devices in use are of the highest standard.
APAC Intracranial Pressure Monitoring Devices Market Segmentation
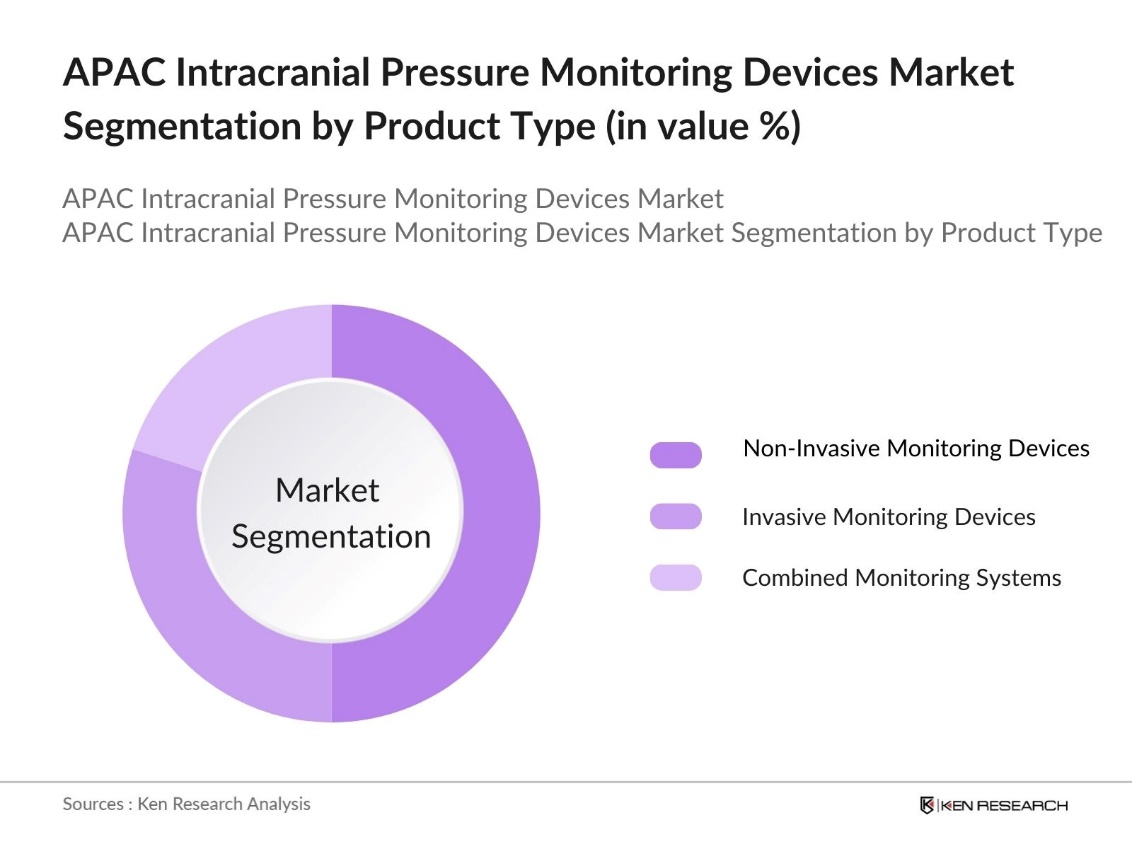
By Product Type: The Asia Pacific Intracranial Pressure Monitoring Devices market is segmented by product type into invasive monitoring devices, non-invasive monitoring devices, and combined monitoring systems. Non-invasive monitoring devices have a dominant market share under the product type segmentation. This is primarily due to the growing preference for less invasive procedures among healthcare providers and patients. Non-invasive devices offer real-time monitoring without the risks associated with invasive methods, such as infections and complications, making them a safer and more appealing choice for hospitals and clinics.
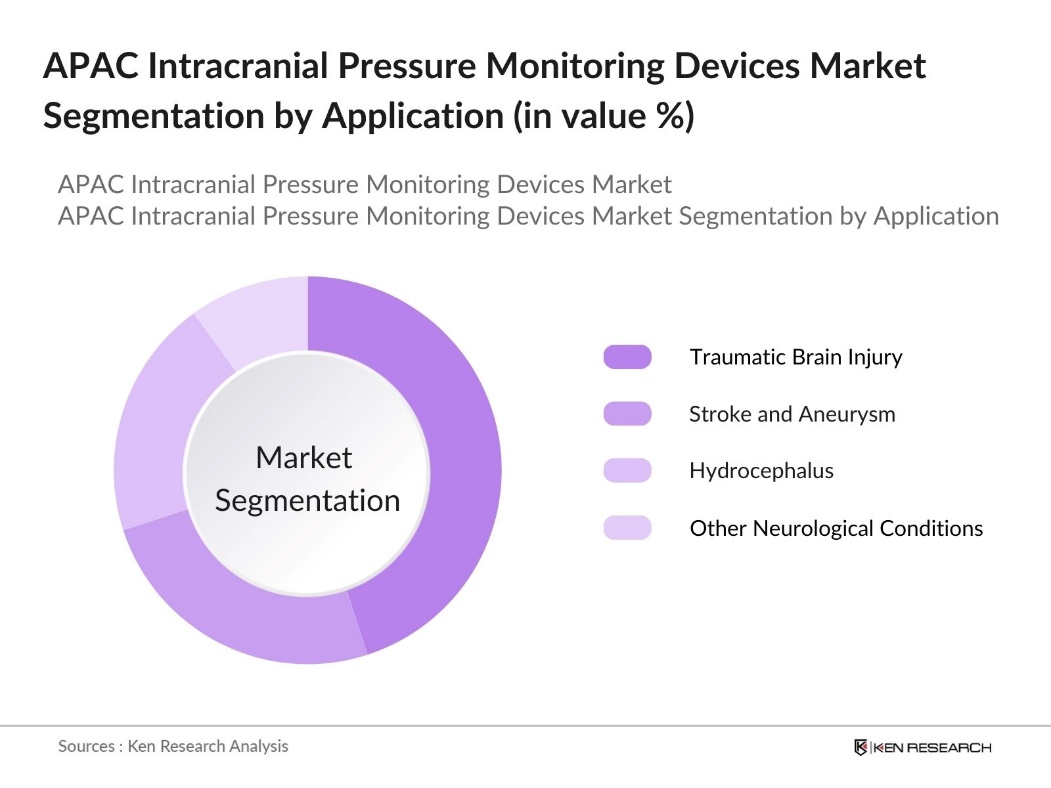
By Application: The Asia Pacific Intracranial Pressure Monitoring Devices market is also segmented by application into traumatic brain injury, stroke and aneurysm, hydrocephalus, and other neurological conditions. The traumatic brain injury segment holds a dominant market share. This can be attributed to the rising number of road accidents and falls, which are major contributors to traumatic brain injuries in the region. Additionally, increasing awareness about the importance of timely intervention in brain injury cases has prompted the use of intracranial pressure monitoring devices, leading to the growth of this segment.
APAC Intracranial Pressure Monitoring Devices Market Competitive Landscape
The market is dominated by several key players, including both local and global companies. These players hold significant influence over the market due to their strong product portfolios, technological advancements, and extensive distribution networks. Major players also engage in strategic partnerships and mergers to expand their market presence and increase their market share.
|
Company |
Establishment Year |
Headquarters |
Key Technologies |
Revenue (USD Mn) |
Employees |
R&D Investments |
Market Share (%) |
Recent Initiatives |
|
Medtronic Plc |
1949 |
Dublin, Ireland |
||||||
|
Integra Lifesciences Corp. |
1989 |
Princeton, USA |
||||||
|
Natus Medical Incorporated |
1987 |
California, USA |
||||||
|
Nihon Kohden Corporation |
1951 |
Tokyo, Japan |
||||||
|
Sophysa |
1976 |
Orsay, France |
APAC Intracranial Pressure Monitoring Devices Industry Analysis
Growth Drivers
- Increasing Prevalence of Neurological Disorders: Neurological disorders such as traumatic brain injuries, stroke, and brain tumors have become a significant health burden in the Asia Pacific region. According to the World Health Organization (WHO), over 60 million people suffer from traumatic brain injuries each year globally, with a large portion in developing APAC nations. The burden on healthcare systems due to these disorders is fostering the demand for intracranial pressure monitoring devices, especially in hospital ICUs.
- Aging Population and Increased Head Trauma Cases: The aging population in the Asia Pacific region has directly contributed to the rise in neurological disorders. Japan, for instance, has one of the largest elderly populations in the world, with recent data indicating that the number of people aged 65 and older reached 36.25 million in 2023. The risk of conditions such as Alzheimers, Parkinsons disease, and head trauma-related incidents is higher in this demographic. This has led to increased adoption of ICP monitoring devices.
- Rising Demand for Non-Invasive Monitoring: Non-invasive monitoring methods are gaining significant traction in the Asia Pacific region due to concerns over the risks associated with catheter-based systems. These devices offer accurate and continuous monitoring without requiring surgical procedures, making them highly appealing for healthcare providers. Countries like China and South Korea are leading the adoption of non-invasive technologies, driven by the increasing focus on patient safety and convenience. Government initiatives promoting the use of advanced medical technologies have further accelerated the shift towards non-invasive solutions in hospitals and healthcare facilities across the region.
Market Challenges
- High Cost of Devices: The high cost of intracranial pressure (ICP) monitoring devices limits their adoption, particularly in developing Asia Pacific countries like India and Indonesia. While advanced systems are more accessible in wealthier nations like Japan and Australia, many healthcare facilities in lower-income regions face financial constraints. This disparity creates challenges for the widespread use of high-cost neurological monitoring equipment in less affluent areas, restricting access to advanced healthcare technologies.
- Limited Awareness Among Healthcare Providers: Awareness and adoption of ICP monitoring devices remain low among healthcare providers in rural and underdeveloped areas of the Asia Pacific region. Many hospitals lack access to modern tools, and a shortage of trained professionals exacerbates the issue. This gap in awareness and resources limits the availability of advanced neurological monitoring solutions, hindering the progress of healthcare services in these underserved regions.
APAC Intracranial Pressure Monitoring Devices Market Future Outlook
Over the next five years, the Asia Pacific Intracranial Pressure Monitoring Devices market is expected to exhibit robust growth driven by increasing healthcare expenditure, growing awareness about neurological disorders, and technological advancements in non-invasive monitoring solutions. With governments across the region focusing on expanding healthcare access and improving healthcare infrastructure, the demand for intracranial pressure monitoring devices is set to increase significantly.
Market Opportunities
- Integration of AI and Big Data for Real-Time Monitoring: The integration of AI and Big Data analytics in intracranial pressure (ICP) monitoring offers promising opportunities for enhancing real-time predictive diagnostics. By utilizing advanced technologies, healthcare professionals can monitor and predict ICP abnormalities more accurately, improving patient outcomes. Countries with well-established healthcare systems, such as Japan, Singapore, and South Korea, are investing in AI-based diagnostic solutions to advance neurological monitoring, supporting more efficient and effective healthcare services.
- Expansion in Emerging Economies: Emerging economies in the Asia Pacific, such as Vietnam, Indonesia, and the Philippines, are experiencing improvements in healthcare infrastructure, creating opportunities for the adoption of advanced medical technologies like ICP monitoring devices. Government initiatives and increased foreign investment are driving the expansion of healthcare services, leading to the establishment of new hospitals and healthcare centers. This growth opens new markets for manufacturers of neurological monitoring devices.
Scope of the Report
|
By Product Type |
Invasive Monitoring Devices Non-Invasive Monitoring Devices Combined Monitoring Systems |
|
By Application |
Traumatic Brain Injury Stroke and Aneurysm Hydrocephalus Other Neurological Conditions |
|
By Technology |
Fiber Optic Devices Microchip Transducer Devices Air-Pouch Devices |
|
By End User |
Hospitals Ambulatory Surgical Centers Specialized Clinics |
|
By Region |
China Japan India Australia South Korea |
Products
Key Target Audience
Neurological Equipment Manufacturers
Medical Device Innovation Hubs and Startups
Medical Equipment Leasing Companies
Health Data Analytics Companies
Government and Regulatory Bodies (e.g., Ministry of Health, China Food and Drug Administration)
Investors and venture capital Firms
Banks and Financial Institutions
Companies
Players Mentioned in the Report
Medtronic Plc
Integra Lifesciences Corporation
Natus Medical Incorporated
Nihon Kohden Corporation
Sophysa
Edwards Lifesciences Corporation
Codman & Shurtleff, Inc.
RAUMEDIC AG
Orsan Medical Technologies
Spiegelberg GmbH & Co. KG
Table of Contents
1. APAC Intracranial Pressure Monitoring Devices Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. APAC Intracranial Pressure Monitoring Devices Market Size (In USD Mn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. APAC Intracranial Pressure Monitoring Devices Market Analysis
3.1. Growth Drivers
3.1.1. Increasing Prevalence of Neurological Disorders
3.1.2. Advancements in Monitoring Technologies
3.1.3. Aging Population and Increased Head Trauma Cases
3.1.4. Rising Demand for Non-Invasive Monitoring (Market specific: Intracranial pressure catheter-based vs. non-invasive)
3.2. Market Challenges
3.2.1. High Cost of Devices
3.2.2. Limited Awareness Among Healthcare Providers (Market specific: Technology adoption in underdeveloped regions)
3.2.3. Regulatory Approvals and Complex Compliance
3.3. Opportunities
3.3.1. Integration of AI and Big Data for Real-Time Monitoring (Market specific: Predictive diagnostics)
3.3.2. Expansion in Emerging Economies
3.3.3. Growing Use in Home-Based Healthcare Monitoring
3.4. Trends
3.4.1. Miniaturization of Monitoring Devices
3.4.2. Wireless and Remote Monitoring Technologies
3.4.3. Increase in Research and Development for Innovative Solutions (Market specific: Wearable monitoring systems)
3.5. Government Regulations
3.5.1. Healthcare Standards for Neurological Monitoring Devices
3.5.2. Regulatory Pathways and Approval Standards (Market specific: FDA approvals in the APAC region)
3.5.3. Government Funding for Neuroscience Research
3.6. SWOT Analysis
3.7. Stakeholder Ecosystem
3.8. Porters Five Forces Analysis
3.9. Competitive Ecosystem
4. APAC Intracranial Pressure Monitoring Devices Market Segmentation
4.1. By Product Type (In Value %)
4.1.1. Invasive Intracranial Pressure Monitoring Devices
4.1.2. Non-Invasive Intracranial Pressure Monitoring Devices
4.1.3. Combined Monitoring Systems
4.2. By Application (In Value %)
4.2.1. Traumatic Brain Injury
4.2.2. Stroke and Aneurysm
4.2.3. Hydrocephalus
4.2.4. Other Neurological Conditions
4.3. By Technology (In Value %)
4.3.1. Fiber Optic Devices
4.3.2. Microchip Transducer Devices
4.3.3. Air-Pouch Devices
4.4. By End User (In Value %)
4.4.1. Hospitals
4.4.2. Ambulatory Surgical Centers
4.4.3. Specialized Clinics
4.5. By Region (In Value %)
4.5.1. China
4.5.2. Japan
4.5.3. India
4.5.4. Australia
4.5.5. South Korea
5. APAC Intracranial Pressure Monitoring Devices Market Competitive Analysis
5.1. Detailed Profiles of Major Competitors
5.1.1. Medtronic Plc
5.1.2. Integra Lifesciences Corporation
5.1.3. RAUMEDIC AG
5.1.4. Natus Medical Incorporated
5.1.5. Codman & Shurtleff, Inc.
5.1.6. Sophysa
5.1.7. Orsan Medical Technologies
5.1.8. Spiegelberg GmbH & Co. KG
5.1.9. Vittamed Corporation
5.1.10. Boston Neurosciences
5.1.11. Edwards Lifesciences Corporation
5.1.12. Elekta AB
5.1.13. Nihon Kohden Corporation
5.1.14. Branchpoint Technologies
5.1.15. Neural Analytics
5.2. Cross Comparison Parameters (Headquarters, Revenue, Employees, Key Technologies, Regulatory Approvals, Market Share, Partnerships, Product Portfolio)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
6. APAC Intracranial Pressure Monitoring Devices Market Regulatory Framework
6.1. Neurological Device Standards
6.2. Compliance and Certification Requirements
6.3. Health Authority Regulations (FDA, TGA, MHLW)
7. APAC Intracranial Pressure Monitoring Devices Future Market Size (In USD Mn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. APAC Intracranial Pressure Monitoring Devices Future Market Segmentation
8.1. By Product Type (In Value %)
8.2. By Application (In Value %)
8.3. By Technology (In Value %)
8.4. By End User (In Value %)
8.5. By Region (In Value %)
9. APAC Intracranial Pressure Monitoring Devices Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Segmentation Analysis
9.3. Marketing and Sales Strategies
9.4. White Space Opportunity Analysis
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
The initial phase involves mapping out all major stakeholders within the Asia Pacific Intracranial Pressure Monitoring Devices market. Desk research, combined with proprietary databases, is utilized to collect industry data. This helps define the variables affecting market dynamics, such as technological advancements and regulatory approvals.
Step 2: Market Analysis and Construction
In this phase, historical data related to intracranial pressure monitoring devices is collected and analyzed. This includes the penetration of devices in hospitals, the ratio of invasive to non-invasive methods, and the overall revenue generated. The analysis focuses on identifying key trends and market shifts.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses are developed and validated through expert consultations, including interviews with professionals from leading medical device manufacturers and hospitals. These consultations provide critical insights into device performance and market demands.
Step 4: Research Synthesis and Final Output
The final step consolidates data from all previous stages and combines it with insights from primary research. This ensures that the report provides a detailed, validated analysis of the Asia Pacific Intracranial Pressure Monitoring Devices market.
Frequently Asked Questions
01. How big is the Asia Pacific Intracranial Pressure Monitoring Devices Market?
The Asia Pacific Intracranial Pressure Monitoring Devices Market is valued at USD 480.5 million, driven by the rising prevalence of neurological disorders and the growing adoption of non-invasive monitoring solutions.
02. What are the key challenges in the Asia Pacific Intracranial Pressure Monitoring Devices Market?
Challenges in the APAC Intracranial Pressure Monitoring Devices market include high costs associated with advanced monitoring devices, limited awareness among healthcare providers, and regulatory hurdles that slow the adoption of innovative technologies.
03. Who are the major players in the Asia Pacific Intracranial Pressure Monitoring Devices Market?
Key players in the APAC Intracranial Pressure Monitoring Devices market include Medtronic Plc, Integra Lifesciences Corporation, Natus Medical Incorporated, Nihon Kohden Corporation, and Sophysa. These companies lead the market due to their strong product portfolios and global presence.
04. What are the growth drivers of the Asia Pacific Intracranial Pressure Monitoring Devices Market?
The APAC Intracranial Pressure Monitoring Devices market growth drivers include the increasing incidence of neurological disorders, rising healthcare expenditures, and advancements in both invasive and non-invasive monitoring technologies, which have improved accuracy and patient outcomes.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















