
USA Bioburden Testing Market Outlook to 2030
Region:North America
Author(s):Yogita Sahu
Product Code:KROD6689
November 2024
94
About the Report
USA Bioburden Testing Market Overview
- The USA Bioburden Testing market, is valued at USD 390 million. This market is primarily driven by stringent regulatory guidelines from agencies like the FDA and ISO 11737, which enforce bioburden control measures for the sterility of medical devices and pharmaceuticals. The growing demand for sterility testing in the production of medical devices and pharmaceutical products has spurred the adoption of bioburden testing protocols, further boosting market demand.
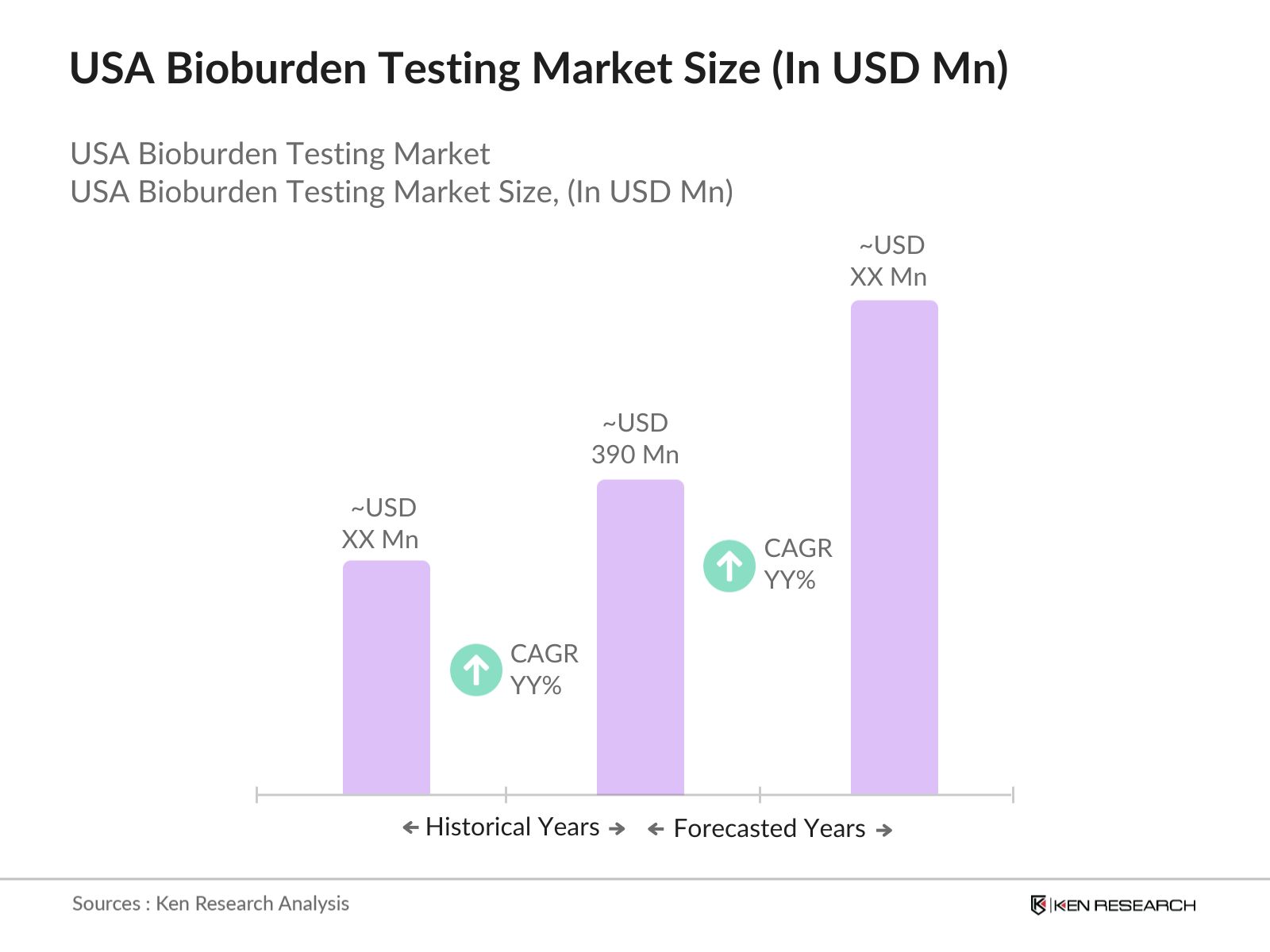
- The market sees dominance in regions like California, Texas, and New York due to the presence of leading pharmaceutical and medical device manufacturers. These states boast advanced healthcare infrastructures, a high concentration of life sciences companies, and strong academic research institutions that support continuous innovation.
- In 2024, the U.S. FDA released updated guidelines on microbial control in sterile pharmaceutical products, mandating more rigorous testing standards for bioburden and sterility. This initiative aims to reduce the risk of contamination and enhance patient safety, driving the demand for more frequent and comprehensive bioburden testing in the pharmaceutical industry.
USA Bioburden Testing Market Segmentation
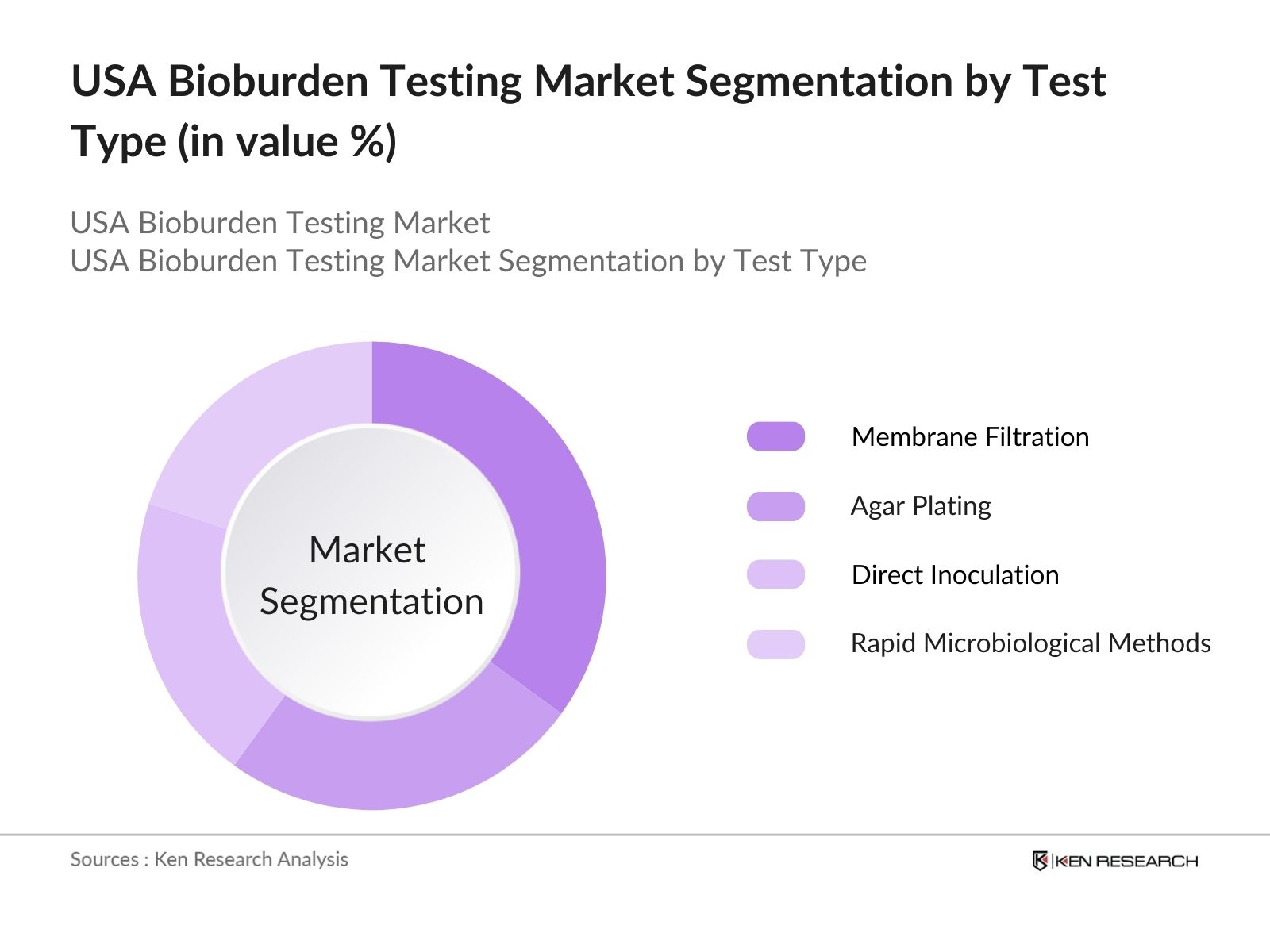
By Test Type: The market is segmented by test type into Membrane Filtration, Agar Plating, Direct Inoculation, and Rapid Microbiological Methods. Among these, Membrane Filtration holds the dominant market share in 2023 due to its reliability and accuracy in detecting and quantifying microbial contaminants in pharmaceutical products and medical devices. Membrane filtration is highly favored by manufacturers in quality control laboratories for sterility testing, especially in liquid-based products.
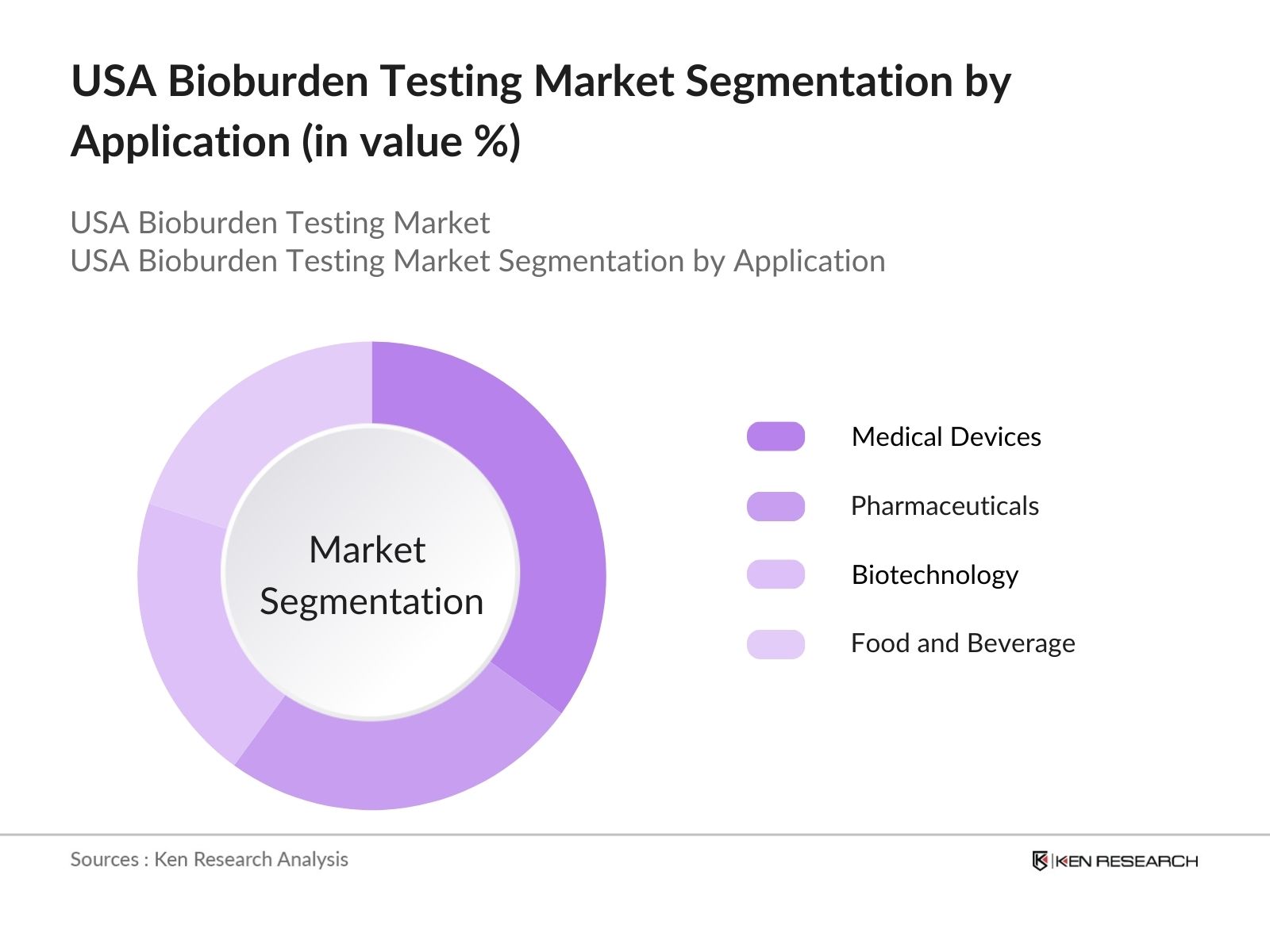
By Application: The market is segmented into Medical Devices, Pharmaceuticals, Biotechnology, and Food and Beverage Industry. The Medical Devices segment has the largest market share in 2023, driven by the rising demand for sterile products in the healthcare sector. This dominance is attributed to strict regulatory standards that mandate bioburden testing across various stages of medical device production, from raw materials to final product release. The increasing complexity of medical devices, such as implantables and surgical instruments, requires thorough microbial testing to ensure patient safety, further bolstering the growth of this segment.
USA Bioburden Testing Market Competitive Landscape
The market is dominated by a mix of global and domestic players. The competitive landscape showcases the presence of both established bioburdens testing service providers and companies offering advanced bioburden testing equipment. These key players drive innovation through partnerships, mergers, and strategic acquisitions.
|
Company Name |
Establishment Year |
Headquarters |
Revenue |
No. of Employees |
Key Products |
Geographical Reach |
R&D Investment |
Client Base |
Key Collaborations |
|
Charles River Laboratories |
1947 |
Wilmington, MA |
|||||||
|
SGS S.A. |
1878 |
Geneva, Switzerland |
|||||||
|
Nelson Laboratories |
1985 |
Salt Lake City, UT |
|||||||
|
Eurofins Scientific |
1987 |
Luxembourg |
|||||||
|
WuXi AppTec |
2000 |
Shanghai, China |
USA Bioburden Testing Market Analysis
Market Growth Drivers
- Increased Pharmaceutical R&D Activities: The market is driven by the rise in pharmaceutical research and development activities. In 2024, pharmaceutical companies in the U.S. invested over $85 billion in R&D, focusing on drug development and clinical trials, particularly in biologics, which require rigorous contamination testing. This demand for sterility and bioburden testing is expected to increase the adoption of advanced testing methods to ensure compliance with FDA regulations for new drug development.
- Regulatory Compliance and Quality Assurance Requirements: The FDA's stringent guidelines for microbial quality control, including sterility and bioburden tests, are a key growth driver. In 2024, the FDA conducted over 1,200 inspections related to good manufacturing practices (GMP) in the biopharmaceutical sector. This regulatory focus on maintaining product safety and preventing contamination has pushed companies to adopt advanced bioburden testing techniques to meet compliance, driving market growth.
- Rising Demand for Medical Devices: In 2024, the U.S. medical device industry produced over 200 million sterilized units, which require strict bioburden testing before they are released to the market. The demand for high-precision medical devices, especially in minimally invasive surgery and diagnostic applications, has resulted in a rise in bioburden testing requirements, as manufacturers strive to meet safety standards for sterile products.
Market Challenges
- Shortage of Skilled Microbiologists: The U.S. healthcare and life sciences sectors are experiencing a shortage of skilled microbiologists and lab technicians. In 2024, it was estimated that the U.S. had over 30,000 unfilled positions for microbiologists, many of which were critical to bioburden testing and quality control in pharmaceutical and medical device manufacturing. This talent gap creates operational bottlenecks for companies trying to scale up testing capacities.
- Complex and Time-Consuming Regulatory Approvals: Companies face delays due to the time-intensive regulatory approval process for new drugs and medical devices, which often involves extensive bioburden testing. The U.S. FDA, in 2024, reported an average delay of 12 months in the approval of medical products due to unmet sterility testing standards. This extended approval timeline challenges manufacturers ability to bring products to market quickly, affecting overall growth.
USA Bioburden Testing Market Future Outlook
Over the next five years, the USA Bioburden Testing industry is expected to see notable growth, driven by advancements in rapid microbial detection technologies and the growing outsourcing of testing services. The demand for sterility assurance in the production of complex medical devices and biologics will further fuel market expansion.
Future Market Opportunities
- Adoption of AI-Powered Testing Solutions Will Accelerate: Over the next five years, the bioburden testing market will see increased adoption of AI-powered solutions for faster, more accurate microbial analysis. By 2029, it is estimated that AI-based testing methods will handle over 50% of all bioburden testing tasks, enhancing efficiency in pharmaceutical and medical device manufacturing processes.
- Increased Focus on Sustainability in Testing Practices: By 2029, bioburden testing will become more sustainable, with companies investing in eco-friendly testing consumables and waste disposal methods. It is projected that 30% of testing labs in the U.S. will adopt environmentally sustainable practices by this time, driven by increasing regulatory pressure and corporate social responsibility goals.
Scope of the Report
|
By Test Type |
Membrane Filtration Agar Plating Direct Inoculation Rapid Microbiological Methods |
|
By Application |
Medical Devices Pharmaceuticals Biotechnology Food and Beverage Industry |
|
By End User |
Pharmaceutical Companies Medical Device Manufacturers Contract Manufacturing Organizations (CMOs) Research Institutes |
|
By Technology |
Conventional Microbiological Testing Automated Testing Rapid Microbial Detection Systems |
|
By Region |
North East South West |
Products
Key Target Audience Organizations and Entities Who Can Benefit by Subscribing This Report:
Pharmaceutical Manufacturers
Medical Device Companies
Biotechnology Firms
Contract Manufacturing Organizations (CMOs)
Hospital and Clinical Testing Laboratories
Investor and Venture Capitalist Firms
Government and Regulatory Bodies (FDA, EPA)
Banks and Financial Institution
Companies
Players Mentioned in the Report:
Charles River Laboratories
SGS S.A.
Eurofins Scientific
WuXi AppTec
Nelson Laboratories
Bureau Veritas
Pace Analytical Services
Microbac Laboratories
ATS Labs
Steris PLC
Table of Contents
1. USA Bioburden Testing Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. USA Bioburden Testing Market Size (In USD Mn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. USA Bioburden Testing Market Analysis
3.1. Growth Drivers
3.1.1. Regulatory Compliance Requirements (FDA, USP, ISO 11737)
3.1.2. Increasing Medical Device Production
3.1.3. Rising Demand for Pharmaceutical Sterility
3.1.4. Innovation in Testing Methods
3.2. Market Challenges
3.2.1. High Costs of Advanced Testing Equipment
3.2.2. Complexity of Regulatory Approval Process
3.2.3. Skilled Workforce Shortage
3.2.4. Limited Adoption of Automation
3.3. Opportunities
3.3.1. Growth in Outsourcing of Bioburden Testing Services
3.3.2. Technological Advancements in Rapid Microbial Detection
3.3.3. Expansion of Medical Device Manufacturing
3.3.4. Integration with Digital Workflow Solutions
3.4. Trends
3.4.1. Automation of Testing Protocols
3.4.2. Adoption of Single-Use Bioburden Testing Systems
3.4.3. Development of End-to-End Sterility Assurance Platforms
3.5. Government Regulation
3.5.1. FDA and ISO 11737 Bioburden Guidelines
3.5.2. USP Chapter <1227> on Microbial Limits
3.5.3. AAMI/ISO Guidelines for Sterilization
3.5.4. Harmonization of International Standards
3.6. SWOT Analysis
3.7. Stakeholder Ecosystem
3.7.1. Bioburden Test Service Providers
3.7.2. Medical Device Manufacturers
3.7.3. Pharmaceutical Companies
3.7.4. Testing Equipment Providers
3.7.5. Contract Research Organizations (CROs)
3.8. Porters Five Forces
3.9. Competition Ecosystem
4. USA Bioburden Testing Market Segmentation
4.1. By Test Type (In Value %)
4.1.1. Membrane Filtration
4.1.2. Agar Plating
4.1.3. Direct Inoculation
4.1.4. Rapid Microbiological Methods
4.2. By Application (In Value %)
4.2.1. Medical Devices
4.2.2. Pharmaceuticals
4.2.3. Biotechnology
4.2.4. Food and Beverage Industry
4.3. By End User (In Value %)
4.3.1. Pharmaceutical Companies
4.3.2. Medical Device Manufacturers
4.3.3. Contract Manufacturing Organizations (CMOs)
4.3.4. Research Institutes
4.4. By Technology (In Value %)
4.4.1. Conventional Microbiological Testing
4.4.2. Automated and Semi-Automated Testing
4.4.3. Rapid Microbial Detection Systems
4.5. By Region (In Value %)
4.5.1. North
4.5.2. Easst
4.5.3. South
4.5.4. West
5. USA Bioburden Testing Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Charles River Laboratories
5.1.2. SGS S.A.
5.1.3. Eurofins Scientific
5.1.4. WuXi AppTec
5.1.5. Nelson Laboratories
5.1.6. Bureau Veritas
5.1.7. Pace Analytical Services
5.1.8. Microbac Laboratories
5.1.9. ATS Labs
5.1.10. Steris PLC
5.1.11. Boston Analytical
5.1.12. Biomerieux
5.1.13. Merck KGaA
5.1.14. Thermo Fisher Scientific
5.1.15. Laboratory Corporation of America Holdings
5.2. Cross Comparison Parameters
Revenue
Number of Employees
Geographical Presence
R&D Investments
Key Clients
Testing Capacity
Partnerships and Collaborations
Market Share
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
6. USA Bioburden Testing Market Regulatory Framework
6.1. FDA Standards and Compliance
6.2. ISO Certification Requirements
6.3. Validation and Verification Processes
7. USA Bioburden Testing Future Market Size (In USD Mn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. USA Bioburden Testing Future Market Segmentation
8.1. By Test Type (In Value %)
8.2. By Application (In Value %)
8.3. By End User (In Value %)
8.4. By Technology (In Value %)
8.5. By Region (In Value %)
9. USA Bioburden Testing Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Research Methodology
Step 1: Identification of Key Variables
In this phase, an ecosystem map was constructed, encompassing major stakeholders in the USA Bioburden Testing market. Extensive desk research, combining secondary databases and proprietary insights, was utilized to identify and define critical market variables.
Step 2: Market Analysis and Construction
Historical data was analyzed to assess market penetration, revenue generation, and key drivers influencing the USA Bioburden Testing market. Key performance indicators, such as service quality statistics and testing capacity, were evaluated to ensure the accuracy of revenue estimates.
Step 3: Hypothesis Validation and Expert Consultation
Hypotheses were developed based on the collected data and validated through expert consultations. Computer-assisted telephone interviews (CATIs) were conducted with industry experts to gain operational and financial insights from key market players.
Step 4: Research Synthesis and Final Output
The final phase involved direct engagement with testing service providers and manufacturers to acquire insights into product segments and consumer preferences. This data was combined with bottom-up analysis to deliver a comprehensive and validated market overview.
Frequently Asked Questions
01. How big is the USA Bioburden Testing Market?
The USA bioburden testing market is valued at USD 390 million, driven by stringent regulatory standards and growing demand for sterility assurance in medical device and pharmaceutical production.
02. What are the challenges in the USA Bioburden Testing Market?
Challenges in the USA bioburden testing market include the high cost of advanced testing equipment, complexity in regulatory approvals, and the shortage of skilled professionals capable of conducting accurate testing procedures.
03. Who are the major players in the USA Bioburden Testing Market?
Key players in the USA bioburden testing market include Charles River Laboratories, SGS S.A., Eurofins Scientific, WuXi AppTec, and Nelson Laboratories. These companies dominate due to their established infrastructure, global reach, and comprehensive service offerings.
04. What are the growth drivers of the USA Bioburden Testing Market?
Growth in the USA bioburden testing market is driven by the increasing production of medical devices and biologics, advancements in microbial detection technologies, and rising outsourcing trends in bioburden testing services.
05. What is the future outlook for the USA Bioburden Testing Market?
The USA bioburden testing market is expected to grow steadily over the next five years, with new technologies, stricter regulations, and growing demand for sterility assurance playing a significant role in shaping its expansion.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















